CleanImplant Research
In addition to establishing a globally recognized "Trusted Quality Mark" for dental implants, the CleanImplant Foundation conducts and supports various research projects. A new key project is the Implant Study 2021-2022, a relaunch of the the previous studies performed in 2014/2015 and 2017/2019. Based on single implant analysis, this new study will again point out differences in the purity of dental implant surfaces.
Other projects focus on the cleanliness of custom made abutments and roughness measurements on dental implants. Future fundamental scientific research into the biologic response of organic and inorganic residues found on sterile packed dental implants complete this area of the CleanImplant Foundation´s work.
Project: Implant Study 2017-2019
On Cleanliness of Sterile Dental Implants - A Global Quality Assessment of Implant Surfaces by SEM/EDS Analysis
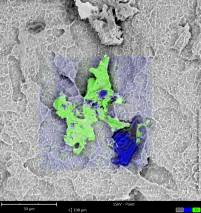
In three consecutive SEM studies conducted by the University Hospital of Cologne and the Charité University Hospital in Berlin, over 250 implants in sterile packaging were analyzed using the same protocol over the past ten years [Duddeck, 2009: Duddeck et al., 2013; Duddeck et al., 2015]*. Results from the most recent study with135 implants and their comparison with previous studies have shown a significant increase in the number of implants with conspicuous residues — despite the fact that it is technically quite possible to produce residue-free implants, which many of the examined implants had proven.
Residue-free medical devices are invariably the result of considerable technological efforts during production and consistent quality management. If, by contrast, adequate quality assurance testing is not performed during production, inferior medical devices are the inevitable result — a fact no formal marketing authorization can ignore..
What´s new in the setup for the Implant Study 2017-2019?
In order to provide a setup which complies with the highest standards of scientific research, the process of analysis will be performed according to DIN EN ISO/IEC 17025. This accreditation not only includes the DIN EN ISO 9001:2015 quality standard but also assessment and monitoring by means of regular inspections carried out by an independent accreditation body in compliance with DIN EN ISO/IEC 17011.
The unpacking of the implants themselves, and the loading of the specimen holders as well as insertion into the SEM will take place in an environment that meets class 100 cleanroom requirements according to United States Federal Standard (US FS) 209 and ISO class 5 according to DIN EN ISO 14644-1. A digitally composed high-resolution SEM image (FSHR) shows the complete surface of an implant at a 120° angle. Thus, the detailed report of every implant shows not only single sections of the sample but always a precise overview of the implant´s surface.
A comprehensive study report will be published in Q1 2021. Preliminary results can be seen in the CleanImplant Newsletter.
Project: Measurement of Implant Roughness
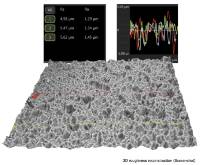
SEM based 3D Roughness Reconstruction vs. White Light Interferometry
With a 3D roughness reconstruction application, based on a „shape from shading” technology, SEM systems areable to generate three-dimensional images and submicrometer roughness measurements. This study compares preciseness and reliability of this method of roughness measurement with white light interferometry. (Doctoral thesis in preparation).