New CleanImplant Guideline released

Dr. med. dent. Dirk U. Duddeck
Founder & Head of Research
Our Mission
More Safety for Your Patients
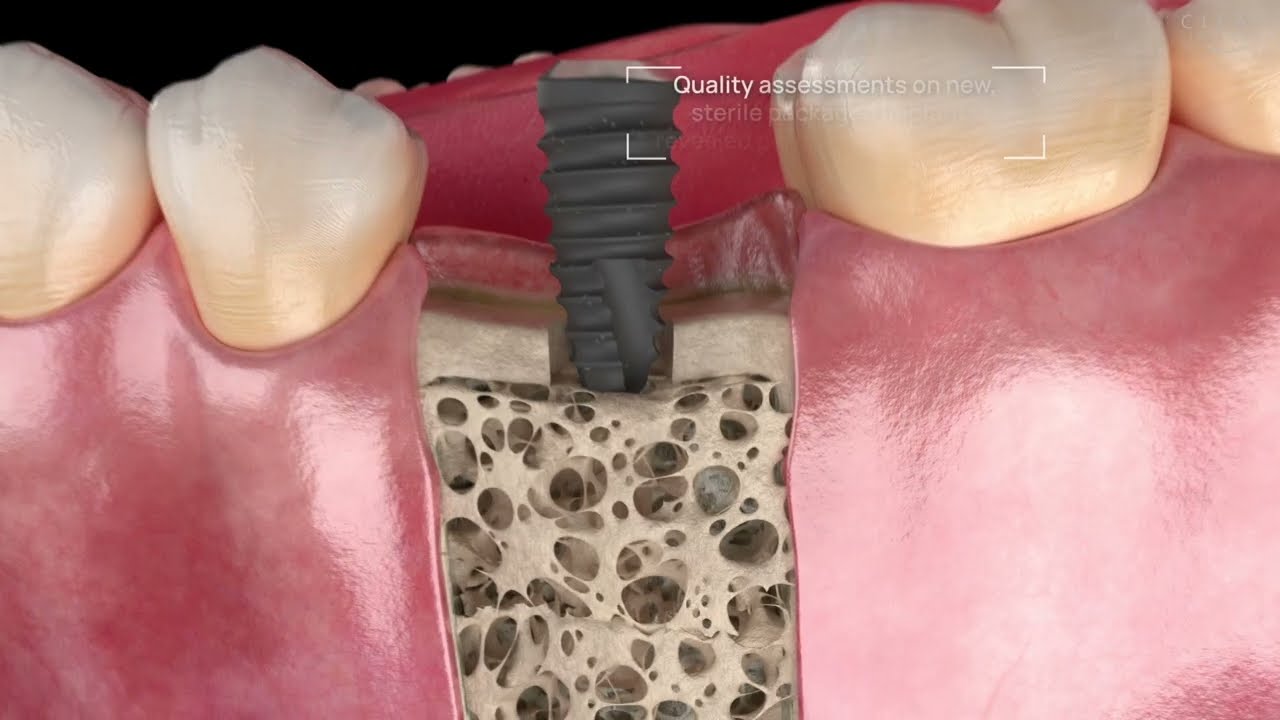
Successful healing of a dental implant is a function of many factors. Nothing is as pivotal for the ‘Osseointegration’ as the sterility and cleanliness of the dental implant itself. After unboxing, every implant must be free of foreign particles and cell-toxic contaminants resulting from deficits in the complex chain of production, packaging and sterilisation. Unfortunately, sterility does not always equate with a clean surface.
Dentists following us on Social Media
Our skyrocketing subscriptions are the result of unbiased implant-related information.
Key Opinion Leaders, Members & Ambassadors
Renowned scientists and practitioners supporting this non-profit initiative actively.
Countries with Project Activities
The awareness campaign for clean implants is reaching dentists worldwide.
Why poor-quality implants
put patients‘ health at risk.
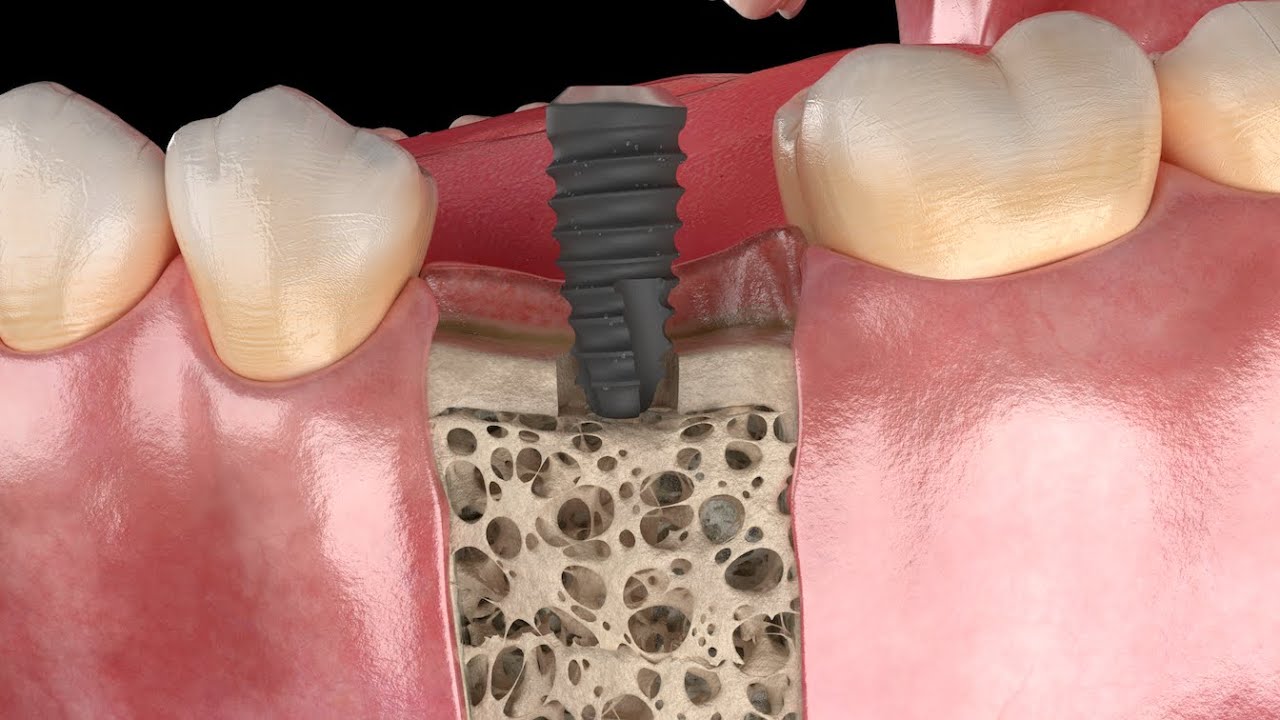
Peri-implantitis is not a rare disease, in severe cases, it can lead to the loss of a newly placed implant. A high inflammatory-bacterial load in the oral cavity often has consequences for the entire organism. The disease, associated with inflammation and bone regression around the implant, has many causes, factory-related contamination such as plastic particles or cytotoxic residues from manufacturing being a primary focus. In our exhaustive studies of implants in the marketplace, significant quantities of contaminants have been found on new, sterile-packaged implants from many manufacturers. The video illustrates why these impurities are a problem.
Facts matter
Thorough testing
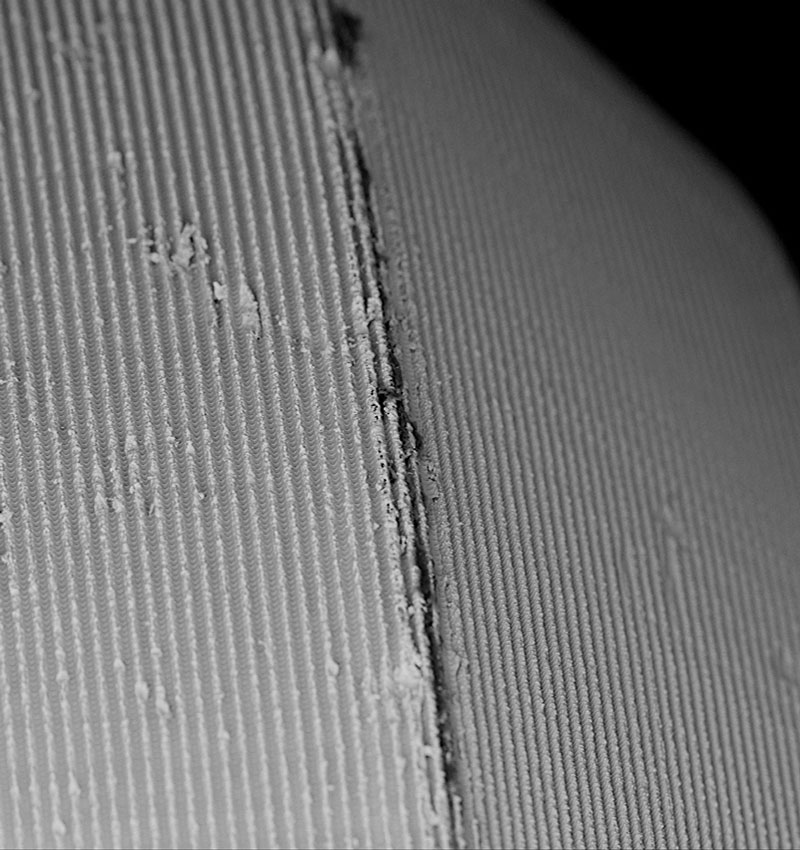
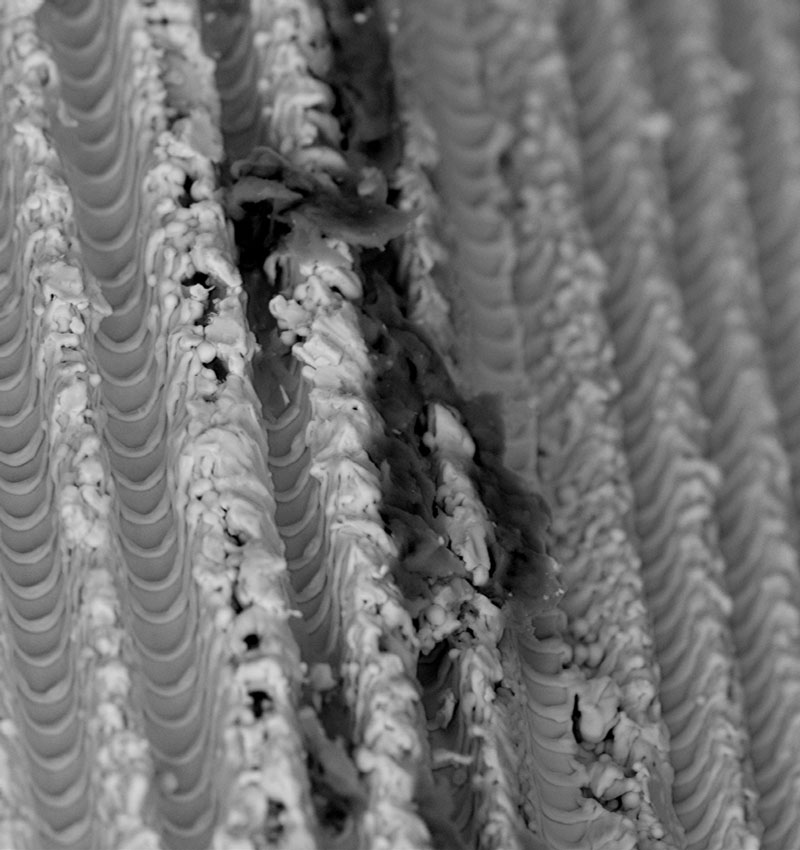
Our studies have detected significant impurities on many sterile packaged and ready-to-use dental implants. These residues are related to unwanted biological consequences associated with peri-implantitis, bone loss, and potentially implant failure. Therefore, patient safety and protection against substandard medical devices are paramount for the CleanImplant Foundation.
Implants reviewed
We have tested implant types of all materials: titanium, titanium alloys, and zirconia.
Implant Brands
Quality assessments on implants from all international manufacturers and countless regional providers.
Years of testing
Benefit from our many years of professional expertise in the field of dental implant evaluations.
Full transparency
Independent Peer-Review
As a non-profit organization, the CleanImplant Foundation operates with rigid standards of transparency. The Foundation commissions exhaustive manufacturer-independent inspections of sterile-packaged implants for surface cleanliness. Comprehensive testing reports provide indications of possible quality deficiencies following cross-batch tests and a strict, non-biased peer review. All tests are exclusively performed in specialized and officially accredited testing laboratories according to DIN ISO standards.
Implant Study 2024/25
Global Quality Assessment of Dental Implants by SEM+EDS
Certified & Approved
Be part of the change
Do you really want to make a difference? Whether you are a dental professional, implant supplier, or contract manufacturer, or you have a product that improves implant healing, controls the osseointegration and stability of implants, or enables more precise planning or positioning, start your journey here!

DENTISTS & CLINICS

TRUSTED QUALITY SEAL

CONTRACT MANUFACTURER CERTIFICATION

APPROVED THERAPY SUPPORT
Dentists & Clinics
Join in – and stand out!
Get exclusive benefits tailored for CleanImplant Certified Dentists and Supporters, and become part of a community of passionate professionals committed to the highest quality standards and ethical integrity.
Elevate your professional profile: From access to cutting-edge implant surface findings and exclusive quality assessment insights to personalized certificates and a direct link to your practice/clinic in our patient directory — the CleanImplant Foundation supports your success in everyday practice. We care for patients — and we make sure they can place their full trust and confidence in your hands.
Join us and stand for uncompromising quality in dental implantology.
- Find a proven clean implant system
- Improve your clinical outcomes
- Get more patients
- Protect your reputation



Trusted Quality Seal
Proof of Excellence
This seal for the outstanding quality of dental implants provides an orientation to practitioners worldwide. The CleanImplant Foundation awards the „Trusted Quality Seal“ to particularly clean implant types only after a comprehensive analysis of randomly selected implant samples followed by a thorough peer-review process. The seal is an expression of the highest quality control in the manufacture of dental implants and ensures an impeachable ethical standard.
- Enhance your quality management
- Empower your brand image
- Increase your customers’ loyalty
- Extend your marketing strategy
CONTRACT MANUFACTURER CERTIFICATION
Implant Manufacturers
Contract manufacturers of dental implants can apply for a unique CleanImplant Certification of Excellence. The coveted “Certified Production Quality” seal proves sustainable high production quality and shows clients that the absolute cleanliness of implants is a top priority from the very start of the manufacturing process.
- Demonstrate your quality management efforts
- Differentiate your production from competitors
- Secure your clients‘ market advantages
- More visibility all over the world



Approved Therapy Support
For dental innovations
The “Approved by CleanImplant” seal provides guidance on the outstanding properties of medical devices, digital technology, software, implant care products or on the functionality of a technology that has been proven in accredited testing laboratories or in a comparable manner to support successful clinical outcomes in the field of dental implantology.
- Increase the accuracy of implant placement
- Accelerate osseointegration for early loading
- Ensure better clinical results
- Provide more safety for your patients
Get latest Information here
Selected CleanImplant News
Media
Videos
Interviews with key opinion leaders and a view behind the scenes.
Meet us
Upcoming Events
Meet the experts responsible for the science supporting the CleanImplant project and receive information about ongoing implant testing.