Welcome to the CleanImplant Foundation
The global initiative for a scientific based view on the implant market aiming for a better, cleaner and safer implantology. Your source of knowledge, background evidence and supportive network.
Selected CleanImplant News:
Congratulation to Dentis: The CleanImplant "Trusted Quality" award for s-Clean SQ-SL
Following the rigorous testing process to evaluate the production quality of the Korean implant manufacturer Dentis, the Scientific Advisory Board of the CleanImplant Foundation agrees to award the "Trusted Quality" seal to the s-Clean SQ-SL implant system after the fulfillment of the requirements for this worldwide recognized award. It confirms the high standard of production and cleanliness of oral implant systems.
Dr. Dirk U. Duddeck, CEO and Head of Research of the CleanImplant Foundation underlines: "Major quality assessment studies reveal too many implants of inferior quality under the scanning electron microscope, contaminated with residues from the production and packaging process. But there are these great companies that set themselves apart in terms of quality with their medical devices and thus meet the quality demands of users and patients. We hope to contribute with our work to a better orientation for dentists and patients to avoid unnecessary risks and foster transparency on the implant market. "
Global Ambassadors' Summit 2023 in Berlin - A view to the future
The international Scientific Board Memebers and CleanImplant Ambassadors were invited to Berlin. Twenty-seven highly esteemed professionals from 15 countries, all experts in the field of implantology, met for the third Ambassadors' Summit at the Adlon Hotel to lend their experience and expertise to the furtherance of the organization's goals and ideals to raise awareness for the need of flawless implant surfaces for a cleaner and safer implantology.
The scientific program was designed with lectures from Prof. Dr. Patrick Schmidlin - University of Zurich, Dr. Birgit Hagenhoff - Tascon GmbH Münster and visiting Professor at University Münster and Dr. Miguel Stanley - CleanImplant Ambassador and Founder of White Clinic Lissabon followed by Dr. Dirk Duddeck who reported and updated about the CleanImplant Roadmap 2.0.
Participating experts:
Prof. Tomas Albrektsson, Sweden; Prof. Anne Wennerberg, Sweden; Prof. Hugo de Bruyn, Belgium; Dr. Luigi Canullo, Italy; Dr. Tudor Cocerhan, Romania; Dr. Dirk Duddeck, Germany; Uli Hauschild, Italy/Germany; Dr. Wim Hobbelink, Netherlands; Dr. Samy Kettinger Hungary; Dr. Jerome Lipowicz, France; Dr. Giuseppe Luongo, Italy; Dr. Michael Norton, UK; Prof. Hakan Ozyuvaci, Turkey; Dr. Bruno Spindler, Germany; Dr. Miguel Stanley, Portugal; Dr. Rita Zeta Gomes, Portugal; Dr. Jin Kim, USA; Prof. Ebru Cal, Turkey; Dr. Saurabh Gupta, India; Prof. Kemal Unsal, Turkey; Dr. Blackie Swart, South Africa; Dr. Frank Maier, Germany; Dr. Amit Patel, UK; Dr. Miltiadis Mitsias, Greece; Dr. Howard Gluckman, South Africa, Dr. Dana Adyani-Fard, Germany.
Welcome Southern Implants !
We took a close look on the implant system INVERTA made by Southen Implant, the leading manufacturer in South Africa. All five samples, randomly selected and thoroughly tested in an accredited laboratory according to DIN EN ISO/IEC 17025, showed a clean surface and met the criteria of the consensus-based CleanImplant Guideline.
The hand-over of the quality certificate was performed at the EAO Meeting in Berlin by Dr. Dirk Duddeck.
Congratulations, Southern Implants!
Dentium successfully passed the strict testing criteria and receives the CleanImplant Trusted Quality award for their SuperLine system
Within the framework of awarding the CleanImplant "Trusted Quality" mark the implant system SUPERLIN made by the Korean manufacturer Dentium was examined with all due care. All five samples, randomly selected, showed a clean surface in the thorough SEM analysis performed by an officially accredited testing laboratory according to DIN EN ISO/IEC 17025. CleanImplant is happy to announce that the SuperLine implant system meets the strict criteria of the consensus-based CleanImplant guideline and was awarded the "Trusted Quality Mark 2023-2024".
Welcome to the family of clean implants!
A new "Trusted Quality" award: Congratulations to ASTRA - Dentsply Sirona
The CleanImplant Trusted Quality awarded to ASTRA TECH EV manufactured by Dentsply Sirona
Following a rigorous testing process to evaluate the production quality of the implant manufacturer Dentsply Sirona, the Scientific Advisory Board of the CleanImplant Foundation awarded the "Trusted Quality" seal to the ASTRA TECH EV implant system. This worldwide recognized award confirms the high standard of production and cleanliness of oral implant systems. Congratulations!
The CleanImplant Foundation announces its new office in New York City
As of September 1st, 2022, Dr. Ken Serota will be the new face of the CleanImplant Initiative in North America.
The CleanImplant Foundation will have a North American office in New York City as of September 1st, 2022. Dr. Ken Serota will act as the Foundation's representative. He will be responsible for bringing the Foundation's information campaign to the profession and the industry to ensure the highest standard and duty of care.
"We are very pleased that Dr. Serota, as a dedicated ambassador of our initiative, will bring awareness of the problem of preventable, manufacture-created contamination of medical devices to the North American dental community. Together with Ken, the Foundation will be able to reach more of our colleagues, as well as implant manufacturers and distributors to foster understanding of the importance of a residue-free implant surface as an indispensable quality criterion," explains Dr. Dirk Duddeck, Managing Director and Head of Research at CleanImplant.
Dr. Serota will represent the CleanImplant Foundation at trade shows, conferences, and congresses. As a speaker, he will educate not only colleagues about the CleanImplant Foundation's study results, their clinical relevance, and the legal implications of using substandard implants. He will be the point of contact for North American implant manufacturers are involved in the CleanImplant quality assessment studies.
Dr. Serota is "… deeply convinced of the CleanImplant Foundation's mission and the unimpeachable scientific standards of its studies. Throughout my career, I have been fascinated by how the synergy of clinical skills, research studies, and ethical standards can ensure that patient centric care is guided by the highest scientific canons of quality control. It is my great pleasure to bring the CleanImplant Foundation as a "Partner in Science" to the profession and the industry in the United States and Canada."
Dr. Serota received his DDS from the University of Toronto Faculty of Dentistry in 1973 with awards in prosthetics. In 1981, he received his Certification in Endodontics and Master of Medical Sciences degree on “radionuclide diagnosis of dental pathology” from the Harvard-Forsyth Dental Center in Boston, Massachusetts under the auspices of Dr. Marjorie Kaplan Jeffcoat. Recognizing the power of online education, he founded the ROOTS Endodontic Forum in 2000 and the interdisciplinary BeiDE-NEXUS Facebook forum in 2015.
His credo is “share knowledge and care about those with whom it is shared”.
Dr. Michael R. Norton: Our patients and ourselves need reassurance
What made one of the world ́s most renowned implant surgeons from London join the CleanImplant Initiative?
"There is a massive need to drive assurance of quality in all medical device fields. The PIP breast implant scandal highlighted the importance and need for tighter regulatory control of medical devices, and the need for device registration so patients can easily be identified when sub-standard or faulty devices are identified. The CleanImplant Foundation is one big step towards this quality assurance program, providing both dentist and patient the reassurance that their implants meet a minimum standard for surface purity and minimal conta-mination. Dentists have to rely on the word of manufacturers and the FDA or CE marks to feel sure that the implants they are using are being manufactured to a standard one would expect of an implantable dental device. Sadly, this is often not the case"...
Read the full interview with Dr. Michael R. Norton, BDS FDS RCS (Ed) on the importance of the CleanImplant Quality Assessment here .
JOMI Publication: Quality Assessment of Five Ceramic Implant Systems
Three zirconia implants revealed significant impurities on their surfaces.
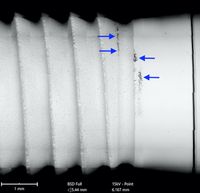
In October 2021, a scientific paper analyzing a total of 25 implant samples from five manufacturers was published in JOMI, the International Journal of Oral & Maxillofacial Implants. The study was initiated by Dirk Duddeck, CleanImplant's head of research, and conducted with renowned scientists such as Tomas Albrektsson, Ann Wennerberg, Florian Beuer, Christel Larsson and Jaafar Mouhyi. The results of this study showed that, in principle, relatively clean implant surfaces are technically possible with ceramic implants, which should, of course, be the aim for all implant materials. The surfaces of the Champions implants (ZV3) and the Z-Systems implants were relatively clean. In contrast, the other investigated surfaces of the vitaclinical, TAV Dental, and ZiBone implants revealed significant contamination on their surfaces. Remarkable was the batch-spanning, packaging-related contamination of the first threads of all (!) tested vitaclinical implants (see figure below). The vast quantities of particles could be identified as polyoxymethylene (polyacetal) in subsequent TOF-SIMS analysis.
This result has striking implications. On the one hand, it can be assumed that these significant amounts of plastic were the initial cause of an uncontrolled foreign body reaction in the event of bone or implant loss. On the other hand, the medical device's packaging does not contain any warning notice for users or patients about the packaging residues, which seems unavoidable from a technical point of view.
A 12-page special print of the study with impressive SEM images of all tested implants can be requested free of charge from the CleanImplant Foundation by email: JOMI-Study_2021@cleanimplant.com
CleanImplant Pilot Study published
One surface characteristic of sterile packaged oral implants is their cleanliness. Oral implants may display different surface impurities of an inorganic or organic nature. These impurities may derive from the manufacturing handling and packaging processes and may remain on the commercially available implant. We are present ly lacking in knowledge of the precise clinical risks of implant impurities. However, contaminations are technically avoidable and, generally speaking, the authors assume all of us would prefer clean implants to avoid potential problems from surface impurities.
The article describes the problem on the implant market and the need for a global quality assessment. Criteria for "clean" implants, as well as the conditions for the globally established "Trusted Quality Mark" are discussed in detail in this paper.
Read the full article here.
2.1 million reports of bad dental implants
June, 2019: The Food and Drug Administration - FDA - has just released two decades of previously hidden data containing millions of malfunctions by medical devices.
This isn't the time to say "we told you so...". However, we should all be concerned, reconsider and double-check our choice of dental implant systems in the future. The question is: Can I blindly trust the information provided by the manufacturer or supplier?
Is the implant system that I use in my dental practice safe and at least free of major impurities? CleanImplant provides precisely this data and valuable information for all members.
When will you join us? Read the full story here.
(Kaiser Health News (KHN) is a nonprofit news service committed to in-depth coverage of health care policy and politics.)
Watch this 6 min video. It may open your eyes as a dental professional!
Interview with Prof. Jack C. Ng, PhD, Diplomate of the American Board of Toxicology, University of Queensland, Brisbane, Australia
Inorganic and organic impurities on sterile packaged dental implants can lead to a foreign body reaction with activation of osteoclasts and a loss of bone in the follow-up. However, this is not the only concern as some of the particles that were found on implants using ToF-SIMS analysis may even have toxic potential. As these particles and impurities are technically avoidable, we should follow the Precautionary Principle, which says: "When an activity raises threats of harm to human health, precautionary measures should be taken even if some cause and effect relationships are not fully established scientifically."
In other words: It´s not our job to prove, that particles shown in this video are a problem for your patients. If an implant manufacturer cannot deliver his medical devices without impurities, it is the job of the manufacturer to give proof, that these impurities cause no harm. CleanImpant provides a stage for implants, that were tested "clean" and particle-free according to the guideline and established consensus.
"The very last thing your patients need right now is a contaminated implant."
Dr. Dirk Duddeck, Implantologist and Founder of the Cleanimplant Foundation, gives answers to current questions.
March, 2020
Prof. Tomas Albrektsson: "We have to study about impurities on implant surfaces!"
Prof. Ann Wennerberg and Prof. Tomas Albrektsson, Members of our Scientific Advisory Board, about their motivation to support the CleanImplant project.
How clean is your implant?
It should be a matter of course that all dental implants are clean when delivered in sterile packaging. Surface pollution with organic particles or significant inorganic residues, originating from the production process are suspected to cause insufficient or missing osseointegration of dental implants. Unintended micrometer-scale particles - particually organic materials such as polyethylene - induce a foreign body reaction with multinucleated giant cells and an osteoclastogenesis that can result in a loss of bone in the early stages of osseointegration.
For more than 10 years we have carried out four consecutive studies in close cooperation with the University of Cologne (Germany) and the Charité University Medicine Berlin (Germany). SEM imaging as well as elemental analyses (EDS) of more than 250 dental implants from over 200 brands were used to establish one of the largest, most comprehensive databases in implant dentistry. Recent analysis in 2018 shows more implants with severe pollution, compared with previous reports.
How many impurities can a patient tolerate?
Alarming remnants and contaminants were found in the SEM-based quality assessment. Areal pollution and particles with iron, copper, chromium, nickel, tungsten, sulfur and large quantities of stainless-steel particles, as well as remnants of polytetrafluoroethylene and other signigficant organic contaminations give cause for concern. However, we also revealed implants with clean surfaces. Read more
One manufacturer of an implant whose analysis showed severe organic contamination and embedded bronze-particles wrote to us that they could "refute the results". They "kindly invited" us "to avoid spreading information that could put a negative image on the company´s reputation".
Another manufacturer claimed that we are "melting the titanium with the energy of the scanning electron microscope" as an explantaion for the implant pollution (ironically, he had no idea how we can reach up to 1.665° C in the SEM). Another accused us of "using different sources of light (!) in the SEM" to produce unfavorable images (ignoring the fact that an SEM needs no light at all). When confronted with poor SEM analysis results, the most disturbing answer was, : "Dentists do not pay attention (for external quality assessments) or even don´t know how to interpret the results that they see." Moreover, as expected: they claimed our "research is biased".
Well, this project is our answer to an "unhealthy state of things" and why there is a need for this global quality assessment of dental implants.
Can dentists trust in the CE label ?
In the video: Dr. Dirk U. Duddeck, Implantologist and Managing Director of the CleanImplant Foundation speaks about possible failings of the European device regulation as studies - years after the PIP scandal - revealed masses of contaminants on sterile packaged dental implants.
A globally recognized label, the CleanImplant "Trusted Quality Mark" is the expression of a high quality level in the cleanliness of dental implants. It will raise the awareness of considerable differences in quality and, even more importantly, give orientation for clean implants which not only practitioners but also patients may trust.
It took years to build up your reputation as an implantogist. Yet, it can be destroyed by one negligent choice of an implant. Stay on the safe side. Choose a proven implant that carries the Trusted Quality Mark. Ask us about the cleanliness of your implant system if it does not carry the mark. Read more
Consensus-based CleanImplant Guideline for "Clean" Implants
In September 2017, objective evaluation criteria for the "Trusted Quality 2017-2018" award were summarized in a consensus paper by the CleanImplant Scientific Advisory Board. The document also contains a process description of the required methods of analysis and gives proof of the CleanImplant project´s transparency.
Click on the image to download the correspondent 4 MByte PDF-file.
News Archive
High production quality of ceramic dental implants deserves to be awarded!
CleanImplant Foundation congratulates Komet Custom Made for the outstanding quality in the production of ceramic dental implants.
CleanImplant is awarding this certification to contract manufacturers, producing implants for various trade brands. The certificate not only confirms high production quality. At least twice a year, the implants' purity is also monitored through unannounced inspections in accredited testing laboratories, using a scanning electron microscope before the final packaging and sterilization process.
The head of research of the foundation, Dr. Dirk Duddeck, assured himself on site about the production processes and handed over the "Certified Production Quality" certificate to the Managing Director Carsten Cieslik and CEO Klaus Rübesamen, who underlines: "As a supplier of ceramic implants, we are really pleased to receive this award from the CleanImplant Foundation. Congratulations to the entire team for the professional implementation of our measures as a full-range supplier in the ceramic segment for dental implantology. The fully comprehensive range of services for our customers has been widened once again by this certification."
Congratulations MegaGen !
After proving the pure and uncontaminated surface of the ANYRIDGE implant system of Korean's MegaGen since 2017, CleanImplant examined also another implant type from the same manufacturer with all due care, the BLUE DIAMOND. The strict process required analyses results of five samples, randomly selected, and a convincing clinical documentation. All results had to meet the criteria of the consensus-based CleanImplant Guideline. After the independent peer-review of two members of the CleanImplant Scientific Advisory Board, the "Trusted Quality 2022-2023" was awarded.
BLUE DIAMOND, welcome to the family of clean implants!
Ambassadors' Summit 2021 - Experts from 16 nations met at Lake Como
In November 2021, members of the Scientific Advisory Board (Tomas Albrektsson, Michal Norton, Hugo deBruyn, Jaafar Mouhyi, and Luigi Canullo) and Ambassadors - influential opinion leaders from 16 nations - accepted our invitation to the second CleanImplant Ambassadors' Summit at Villa Geno on Lake Como. In his lecture, Prof. Tomas Albrektsson of the Sahlgrenska Academy in Gothenburg emphasized in his presentation the need and requirement for implants to be sterile when they leave the factory and, of course, be free of foreign particles. Factory-related carbonaceous particles are suspected to be responsible for uncontrolled foreign body reactions. Residues of foreign metals also harbor the risk of undesirable corrosion.
Prof. Hugo de Bruyn, University of Nijmegen, presented preliminary results of a study analyzing the presence of metal particles in peri-implant sulcus fluid in correlation to the respective peri-implantitis status.
"How did dental implants become the most common medical device reported as failing to the FDA?" Madris Tomes, data analyst and former FDA employee reported live from Pennsylvania and helped interpret the skyrocketing FDA reports of oral implant losses. It appears that not only compromised patients or poorly trained dentists but also the high number of factory-contaminated dental implants are responsible for the high number of reported implant losses.
Dr. Dirk U. Duddeck, head of research at the foundation, summarized the results of a recent benchmark study of more than 100 implants in his presentation: "More than 30 percent of all previously sterile-packaged samples had significant contamination that could have been avoided with proper quality management." Dentists may unknowingly use these implants, putting not only patients' health at risk but also themselves, for there are also legal implications, including claims for damages from the patient side.
The participants discussed strategies for supporting manufacturers who supply uncompromising medical devices and how the foundation can defend itself against increasing threats of legal action from manufacturers who offer implants of inferior quality. In the future, particular attention will be paid to those manufacturers who have failed to improve their quality management and implant production for years despite being informed of significant impurities on their implants. Instead, some have verbally attacked the scientists of the CleanImplant Foundation and even personally threatened them.
The short video linked gives an impression of this extraordinary expert meeting.
Welcome to the family of clean implants!
The CleanImplant "Trusted Quality" award was presented to the CEO Dr. Ulrich Volz of Swiss Dental Solutions for the SDS2.2 !
The Swiss manufacturer of ceramic implants Swiss Dental Solutions has been recognized for its quality following an independent peer review process. On October 15, 2021, the certificate, signed by two members of CleanImplant's Scientific Advisory Board, was ceremoniously presented to CEO Dr. Ulrich Volz on the occasion of the first JOINT CONGRESS for CERAMIC IMPLANTS JCCI in Kreuzlingen, Switzerland.
The strict CleanImplant criteria of the consensus-based guideline were fully met by five different implant samples whose analyses reports eventually passed the independent peer-review. Dr. Dirk Duddeck, Managing Director and Head of Research of the CleanImplant Foundation, congratulates Dr. Ulrich Volz on the proof on this uncompromising surface quality of the implant system 2.2.
"Fabrikneue Implantate - Steril verpackt und schon verunreinigt?"
Webinar mit Dr. Dirk U. Duddeck (German language)
Weder die Ankündigung rechtlicher Schritte gegen die Veröffentlichung von Analyseergebnissen noch telefonische Bedrohungen gegen die untersuchenden Wissenschaftler ändern etwas an dem Befund: Eine aktuelle Studie zur Qualitätsbeurteilung steril verpackter Implantate, die in Zusammenarbeit mit der Charité – Universitätsmedizin Berlin durchgeführt wurde, deckte unter den mehr als 100 untersuchten Proben eine alarmierende Anzahl von Zahnimplantaten mit werksseitigen Verunreinigungen auf.
Die Folge: Im Vertrauen auf das Qualitätsversprechungen von Implantatherstellern verwenden Zahnärzte oftmals unwissentlich Implantate mit erheblichen Restverschmutzungen, die bei Patienten zu ungewollten Fremdkörperreaktionen bis hin zu Periimplantitis führen können. In Interviews mit renommierten Experten wie Prof. Tomas Albrektsson (Department of Biomaterials, Universität Göteborg) und Prof. Jack Ng (American Board of Toxicology, Universität Queensland) wird die Gefahr minderwertiger Medizinprodukte am Beispiel kontaminierter Implantate für Patienten unterstrichen. Das Webinar verdeutlicht Art und Ausmaß der Befunde, beschreibt klinischen Folge für Patienten und juristische Implikationen für Behandler, zeigt aber auch, wie solche Risiken vermieden werden können.
IDS 2021: Dental Implant Quality under Scrutiny
On-site SEM analysis of implants for dentists and demos for implant manufacturers at IDS 2021 in cooperation with Thermo Fisher Scientific
"Sterile packaged" does not mean an implant is necessarily free of contaminants. This disturbing realization is slowly dawning on dentists not only in Europe and the US. Exceeding 100.000 subscriptions on Facebook’s CleanImplant site, the number of dentists worldwide intrigued by this revelation increased tenfold over the past two years.
Transparent Testing Procedure
At the International Dental Show IDS in Cologne in September 2021, the non-profit CleanImplant Foundation based in Berlin, Germany, once again showcases the quality check of dental implants using a scanning electron microscope. Installed exclusively for this event in Hall 10.2 in collaboration with Thermo Fisher Scientific and the 'Medical Materials Research Institute', the set-up provides full transparency of the Foundation's quality assessments.
Dentists and manufacturers alike can witness the meticulous and independent quality check of dental implants in detail, from start to finish. The open and public demonstration allows spectators to learn about the extent of factory-related contamination of sterile packaged implants, and most importantly, its direct consequences. The SEM unveils whether an implant meets the strict consensus-based CleanImplant Quality Guidelines, and dentists are encouraged to participate in finding out whether what they deem the implant system used in their practice is actually safe. See the video here!
A new "Trusted Quality" award: Congratulations to Biotech Dental
A further CleanImplant Trusted Quality award for the dental implant system Kontact S produced by Biotech Dental
Following a rigorous testing process to evaluate the production quality of the French implant manufacturer Biotech Dental, the Scientific Advisory Board of the CleanImplant Foundation awarded the "Trusted Quality" seal to the Kontact S implant. This worldwide recognized award confirms the high standard of production and cleanliness of oral implant systems.
"Congratulations to Biotech Dental for its uncompromising implementation of CleanImplant's quality requirements", Dr. Duddeck, CleanImplant Founder and Head of Research underlined. "Major quality assessment studies show too many implants of inferior quality under the scanning electron microscope, contaminated with residues from the production or packaging process. But there are these great companies that set themselves apart in terms of quality with their medical devices and thus meet the quality demands of users and patients. The CleanImplant award for the Kontact S implant system is well deserved. We hope to contribute with our work to a better orientation for dentists and patients to avoid unnecessary risks and foster transparency on the implant market".
A new award for high production standard of ceramic dental implants
CleanImplant Foundation congratulates CeramTec, the Ceramic Experts !
On January 6, 2021, the CeramTec Group, a world innovation leader for advanced ceramics, was awarded the "Certified Production Quality" seal by the CleanImplant Foundation in Berlin. Based on the globally established CleanImplant consensus guideline on the cleanliness of dental implants, CleanImplant is awarding a certification to contract manufacturers, producing implants for various trade brands. The certificate not only confirms high production quality. At least twice a year, the implants' purity is also monitored through unannounced inspections in accredited testing laboratories, using a scanning electron microscope before the final packaging and sterilization process.
"We only deliver ceramic implants to our customers that meet the very highest quality standards and are truly clean," emphasizes Dr. Hadi Saleh, CEO of the CeramTec Group. "We are pleased to be able to prove this with the current award."
CleanImplant Webinar
Fabrikneue Implantate
Steril verpackt und schon verunreinigt?
Click on the image for a preview or here to see the full version.
Webinar mit Dr. Dirk U. Duddeck (German language)
Weder die Ankündigung rechtlicher Schritte gegen die Veröffentlichung von Analyseergebnissen noch telefonische Bedrohungen gegen die untersuchenden Wissenschaftler ändern etwas an dem Befund: Eine aktuelle Studie zur Qualitätsbeurteilung steril verpackter Implantate, die in Zusammenarbeit mit der Charité – Universitätsmedizin Berlin durchgeführt wurde, deckte unter den mehr als 100 untersuchten Proben eine alarmierende Anzahl von Zahnimplantaten mit werksseitigen Verunreinigungen auf.
Die Folge: Im Vertrauen auf das Qualitätsversprechungen von Implantatherstellern verwenden Zahnärzte oftmals unwissentlich Implantate mit erheblichen Restverschmutzungen, die bei Patienten zu ungewollten Fremdkörperreaktionen bis hin zu Periimplantitis führen können. In Interviews mit renommierten Experten wie Prof. Tomas Albrektsson (Department of Biomaterials, Universität Göteborg) und Prof. Jack Ng (American Board of Toxicology, Universität Queensland) wird die Gefahr minderwertiger Medizinprodukte am Beispiel kontaminierter Implantate für Patienten unterstrichen. Das Webinar verdeutlicht Art und Ausmaß der Befunde, beschreibt klinischen Folge für Patienten und juristische Implikationen für Behandler, zeigt aber auch, wie solche Risiken vermieden werden können.
For the first time CleanImplant Foundation awards a ceramic implant system:
BioWin! - Champions-Implants
Dental practitioners have to consider that there are differences in the production quality of titanium-made implants AND ceramic implants. After the thorough testing procedure of five BioWin! implant samples (identical to ZV3/Standard Zirkon Implantat) and the evaluation of the clinical documentation, two members of the CleanImplant Scientific Advisory Board independently confirmed in a peer-review the coveted award be issued!
Congratulations Champions-Implants!
Congratulations CAMLOG !
Within the framework of awarding the CleanImplant "Trusted Quality" mark we have examined the implant system CONELOG made by the manufacturer Camlog from Germany with all due care. All five samples, randomly selected, showed a clean surface in the thorough SEM analysis performed by an officially accredited testing laboratory according to DIN EN ISO/IEC 17025. We are happy to announce that the CONELOG implant system meets the strict criteria of the consensus-based CleanImplant guideline and has been awarded the "Trusted Quality Mark 2020-2022". Welcome to the family of clean implants!
Welcome Sweden & Martina !
We took a close look on the implant system PRAMA made by Sweden & Martina, a leading manufacturer in Italy. All five samples, randomly selected, showed a clean surface in the thorough SEM analysis performed by an officially accredited testing laboratory. We are happy to announce that the PRAMA implant system meets the strict criteria of the consensus-based CleanImplant guideline and was awarded with the "Trusted Quality Mark 2020-2021". Congratulations!
Find more information and impressive SEM images of the PRAMA implant system here.
Trusted Qualitiy Mark for In-Kone from Global D
2019, October| During the EAO Meeting in Lisbon, Dr. Dirk Duddeck handed over the Certificate of CleanImplant "Trusted Quality" to the management of Global D, the French manufacturer located in Lyon. After the thorough testing procedure of five implant samples and the evaluation of the clinical documentation, two members of the CleanImplant Scientific Advisory Board confirmed in a peer-review independently to issue the coveted award!
Feedback from Colleagues
"I am a dentist in private practice in Johannesburg , South Africa. I recently attended your lecture at the SAOO congress in Durban.
I must both thank you and compliment you on the lecture which was delivered with much humour and academic excellence, and I must commend you on your research on such a difficult and sensitive topic! Prior to your lecture I had, probably like most of my colleagues, assumed that all implants on the market were of the highest standard of cleanliness. How wrong I have been!"
Mike Holmes, South Africa
"I attended your lecture in Skopje today and I was very positively surprised by all the information you shared with us. This is a game changer in the implant business. I hope you get your work published. I think we (dentists) do not pay enough attention in the surface quality since we blindly trust the manufacturers once they gain the CE stamp."
Gorazd Danilovski, Slovenia
Read more from other colleagues here...








_Seite_01.jpg/picture-200?_=16d2f8a2670)